Fast and High Efficiency HPLC Separations Using Sub-2µm Particles

As chromatographers, whether we’re working in the early stages of column screening or in the latter stages of method development and optimization, we often wish that our separations were:
- faster,
- more efficient with higher plate counts so we can achieve better peak resolution, or
- faster and more efficient.
In this blog, we’ll discuss the potential for using columns packed with sub-2 µm fully porous particles to achieve those goals.
To get started, let’s think about what is happening inside an HPLC column during the separation of two compounds. First, at the moment we inject our sample mixture onto the head of a column, we can think of the two compounds as existing in two separate narrow bands that are perfectly overlapping. Then, as liquid mobile phase is pumped, both bands are swept down the length of the column. Sample molecules partition from the mobile phase into the column stationary phase and back out into the mobile phase many, many times. Depending on their relative physical and chemical characteristics, one of the compounds might spend more time in the stationary phase during each of those partition events than the other compound. This behavior can be described by each compound’s retention factor (k’), which is the average amount of time that each compound spends in the stationary phase versus the time it spends in the mobile phase. Provided that the retention factors of the two compounds are different, it is theoretically possible to separate them. It sounds simple enough, but there is another important factor to consider: column efficiency.
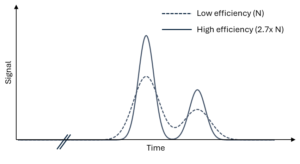
Figure 1 – Overlays of theoretical peaks observed using two different columns. With the low-efficiency column, peaks are not baseline resolved.
As we mentioned, compounds with different retention factors will eventually separate from one another as they travel the length of an HPLC column. During that process, however, several phenomena related to flow and diffusion cause each of our compound bands to spread out and get wider. This is commonly called “band broadening,” and the extent of that broadening as a function of column length is referred to as “column efficiency.” HPLC columns that have relatively low efficiencies might separate two compounds in the sense that their retention factors are quite different, but the detected peaks might still significantly overlap because each of the bands is so broad. By using a column with higher efficiency, peaks will remain narrower during the separation process and will be better resolved from one another at the time we detect them. To illustrate this, Figure 1 shows theoretical peaks for two compounds separated using low- and high-efficiency columns. While the retention factors on both columns are the same, higher column efficiency results in better peak resolution. There are several ways to achieve higher column efficiencies, but let’s consider the one we mentioned at the beginning of the blog: columns packed with sub-2 µm fully porous particles.
For several decades, it has been very common to perform HPLC separations using columns packed with 5 µm fully porous particles and, in many cases, separation performance is perfectly acceptable.
Sometimes, however, an analyst may need a bit more resolution between their compound peaks or they may desire to have a faster runtime. In these cases, a column packed with smaller particles might offer a good solution. Let’s consider a few scenarios.
Scenario 1
Suppose we have two different HPLC columns of the same length. One is packed with 5 µm particles and the other is packed with 1.8 µm particles. The column with smaller particles will have a column efficiency that is roughly 2.7-times higher, and, all else being equal, it will provide 1.6-times higher peak resolution values. This is a great improvement in the quality of the separation, but since the columns are the same length, it might not necessarily translate to faster runtimes.
Scenario 2
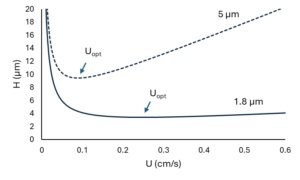
Figure 2 – Theoretical performance curves (H vs U) for 5- and 1.8 µm particles.
We again have two columns: a 25 cm column packed with 5 µm particles and a 10 cm column packed with 1.8 µm particles. The longer and shorter columns will provide the same approximate column efficiencies (i.e. plate count, N), but, all else being equal, the runtime will be 2.5-times shorter with the 10 cm column packed with 1.8 µm particles. In this case, there is no improvement in peak resolution as there was in Scenario 1, but there is great improvement in speed.
At this point, we should point out that these two scenarios don’t present the complete picture regarding the advantages of small particles. In reality, we don’t need to choose between better efficiency and faster runtimes; we can have both simultaneously! To understand how, let’s look at the respective theoretical performance curves for 5- and 1.8 µm particles as shown in Figure 2. From this plot of plate height (H) versus mobile phase linear velocity (U), we’ll highlight three features.
1) The curves for both 5 and 1.8-µm particles have the same general shape. At low mobile phase linear velocities, plate heights are relatively high. As we move to faster velocities, plate heights decrease until they reach their respective minimum values. The point at which this occurs is called the optimum linear velocity (Uopt). Then, at even faster velocities, plate heights increase again.
2) Compared to 5 µm particles, plate heights for 1.8 µm particles are lower over the entire range of mobile phase linear velocities. We also see that the minimum plate height for the smaller particles occurs at a faster linear velocity (Uopt) than it does for the larger particles. This shows that columns packed with smaller particles should indeed be operated at faster velocities than columns packed with larger particles.
3) At faster mobile phase linear velocities, the slope of the H vs. U curve is shallower for smaller particles. This means that we can operate columns packed with small particles well-beyond Uopt without severe detriment to column efficiency.
With these points in mind, we can consider one more scenario:
Scenario 3
Once again, we have two columns: a 15 cm column packed with 5 µm particles and a 10 cm column packed with 1.8 µm particles. Suppose we operate the first column at its optimum flow rate and achieve a column efficiency of 15,000 plates. If the retention factor (k’) of the last peak is ~ 3, our total runtime will be about 10 minutes. If we operate the second column packed with smaller particles at its optimum flow rate, we predict that we will achieve a column efficiency of 27,000 plates in a runtime of just 2.7 minutes. The column packed with 1.8 µm particles provides both higher efficiency and faster runtimes – an ideal situation!
Okay… So what’s the catch? (There’s always a catch, right?) Well, in the case of sub-2 µm particles, it’s the pressure required to operate the column. In Scenario 3, the 10 cm column packed with 1.8 µm particles needs 14-times higher pressure than the 15 cm column packed with 5 µm particles. Perhaps we don’t have a UHPLC instrument capable of providing those pressures in our lab. Or maybe we are concerned about column lifetime if we are routinely running under such demanding conditions. Is there an alternative approach for improving efficiency and speed that does not require such high pressures? Yes! Superficially porous particles provide such an option, and we will discuss them very soon in our next blog. Stay tuned!
Additional Information and Resources
- Download or request a copy of our Chiral Handbook for HPLC and SFC applications here.
- Check out our chiral database with over 900 chiral applications
- Download the Chiral Stationary Phases brochure for more information about our chiral columns Chiral HPLC & SFC Columns.
