6 Top Chiral Chromatography Questions


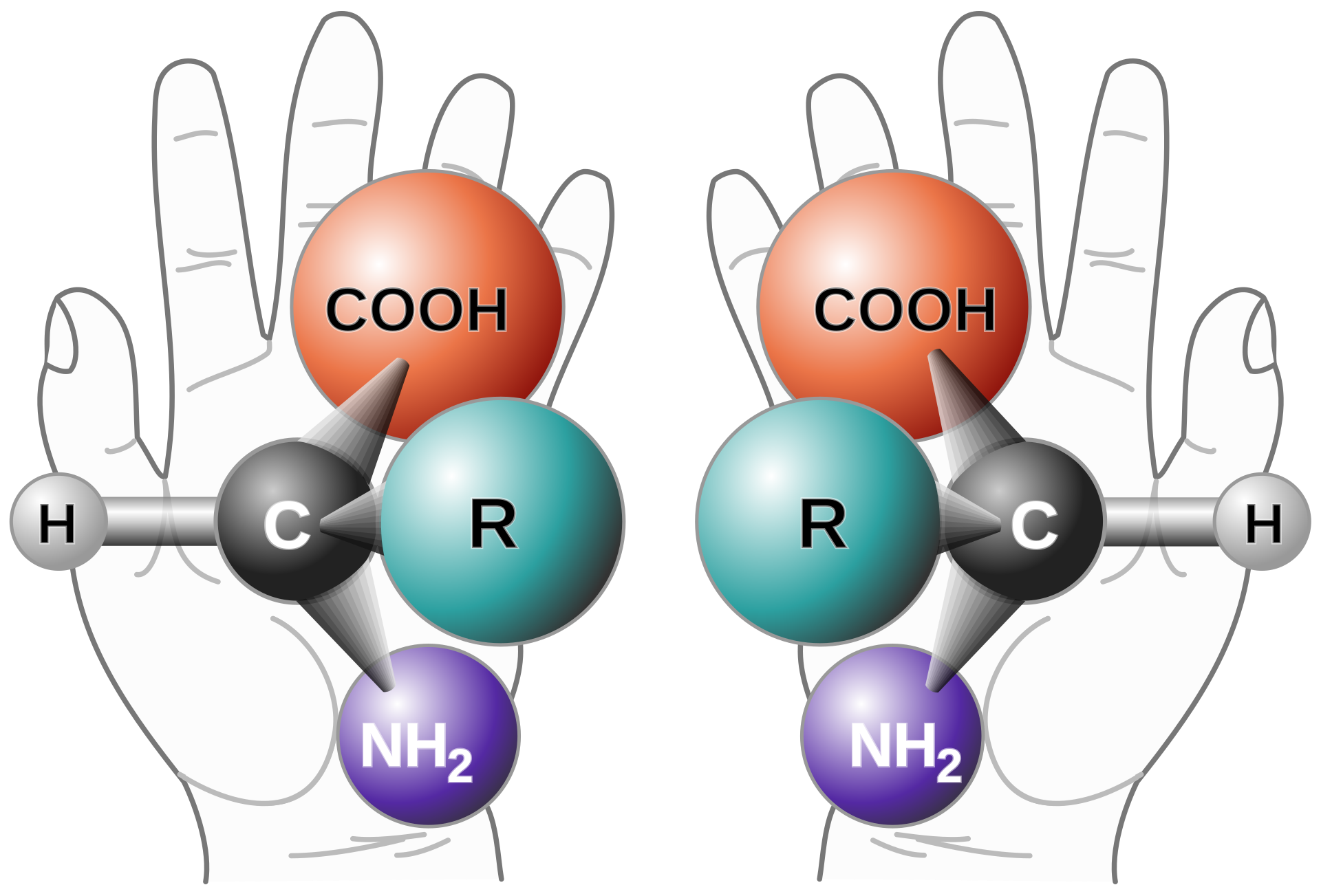 A chiral molecule is one in which the stereoisomers are mirror images of each other. These mirror images – an example of molecular symmetry – are referred to as enantiomers (see image at right – courtesy of Wikipedia).
A chiral molecule is one in which the stereoisomers are mirror images of each other. These mirror images – an example of molecular symmetry – are referred to as enantiomers (see image at right – courtesy of Wikipedia).
From a separation standpoint, chiral molecules pose unique challenges. Because they are chemically and physically identical and only differ in their spatial arrangements, enantiomers will typically coelute in chromatographic separations that use common achiral stationary phases, like C18, phenyl-, cyano, etc.
But because different enantiomers can sometimes interact with other chiral molecules (like protein receptors) in very different ways, gaining insight into chirality during separations is critically important in pharmaceutical analysis. Unlike normal or reversed-phase non-chiral separations, however, there is no universal chiral stationary phase (CSP), and chiral separations do not rely solely on hydrophobicity.
 Chiral separations rely on the differences in orthogonality and planar shape between the two stereoisomers and their interactions with the carefully chosen stationary phase to form transient diastereomeric complexes that are the basis for their different retention times.
Chiral separations rely on the differences in orthogonality and planar shape between the two stereoisomers and their interactions with the carefully chosen stationary phase to form transient diastereomeric complexes that are the basis for their different retention times.
The key challenge with chiral separation typically involves column selection and separation methodology. Column selection is critical, as even seemingly small differences between CSPs can impact selectivity and specificity. Often, more than one type of column may provide some separation, but in other cases, only one or two CSPs will provide adequate resolution of the enantiomers.
Because we’ve been a world leader in HPLC and SFC chiral separations since 1972, we’ve received and answered many questions regarding:
- chiral separation column selection
- mobile phase and co-solvent selection
- method optimization.
Here are answers to six of the most common questions we’ve encountered.
![]()
Q: What are some common chiral stationary phases for chiral compounds?
A: With non-chiral separations, the C18 stationary phase is widely used – across an extensive range of molecules and conditions. Chiral separations, however, differ considerably. These are the 3 stationary phases which cover a broad range of chiral compounds:
- Pirkle-type columns
For a wide range of class compounds (Regis Whelk-O columns and other Pirkle-Type chiral stationary phases) - Polysaccharide columns
The most common column type to separate enantiomers (Regis Reflect columns) - Crown-Ether columns
For separating amino acids and compounds containing primary amines (Regis Chirosil columns)
Q: Where can I find specific method information for chiral applications?
A: We’ve created a library of over 950 chiral applications. Each one has method information for a range of column types and separation modes. Visit the library here.
Q: Can I dilute my sample in a solvent other than the mobile phase?
A: Ideally, samples should be dissolved in mobile phase. In cases where this is not possible – for example, if the sample is not fully soluble in the mobile phase – care should be taken when injecting a sample dissolved in a stronger solvent than mobile phase since precipitation can occur. One way solubility can be improved is through the use of dichloromethane (DCM) mixtures.
Q: Can I use the same column for reversed-phase and normal phase solvent systems while doing method development?
A: All Regis CSPs can be used with both normal and reversed-phase solvents – but there are some restrictions for coated polysaccharide phases. Generally, Pirkle-Type CSPs will yield better separations in normal phase mode, but there are also numerous examples of separations in reversed-phase mode that outperformed normal phase mode.
 Q: Which chiral column is right for my separation?
Q: Which chiral column is right for my separation?
A: Chiral separation is difficult to predict. To determine the best column and method, it’s best to have a chiral screening conducted by experts. Regis can help identify the best column for your chiral separation with our free screening service. A chiral separations expert will identify the best method suited for your separation. Click to get started or to learn more (https://www.registech.com/free-chiral-screening)
If you are planning to do your own method development, we recommend following a specific sequence of Regis columns. For example, when doing method development in-house, the first choice is always the Whelk-0 1, which exhibits a broad range of selectivity and has the ability to invert elution order if needed. (To learn more about the preferred sequence, download or request a free digital or hard copy of our 2nd edition chiral handbook.)

Q: Are there any restrictions on the types of solvents I can use with the chiral stationary phase (CSP)?
A: There may be – it depends on the specific CSP you are using. For example, with Regis’ coated polysaccharide phases, there are some restrictions – solvents that swell or dissolve the phase must be avoided (for example, dichloromethane, chloroform, tetrahydrofuran, ethyl acetate, acetone, etc.) With Regis’ Whelk-O 1 and immobilized polysaccharide phases there are no solvent restrictions.
![]()
 Interested in learning more about Regis Technologies’ chromatography columns for both chiral and achiral separations? Here’s a video featuring Ed Franklin Ph.D., a Chromatography Applications Scientist at Regis, discussing just a few of the technical resources Regis makes available to chromatography users. (Watch it here.)
Interested in learning more about Regis Technologies’ chromatography columns for both chiral and achiral separations? Here’s a video featuring Ed Franklin Ph.D., a Chromatography Applications Scientist at Regis, discussing just a few of the technical resources Regis makes available to chromatography users. (Watch it here.)
 You can also request a free digital or hard copy of our 2nd edition chiral handbook. The second edition of the Chiral Handbook provides nearly 400 pages of information related to chiral separations. Sections include HPLC and SFC Method Development Guides, frequently asked questions, and column selection advice.
You can also request a free digital or hard copy of our 2nd edition chiral handbook. The second edition of the Chiral Handbook provides nearly 400 pages of information related to chiral separations. Sections include HPLC and SFC Method Development Guides, frequently asked questions, and column selection advice.
Additional Resources
► Regis Chiral Columns for HPLC and SFC