Getting Started with Chiral Method Development Part Two: Finding a CSP


In a previous blog, we discussed the concept of molecular chirality and its importance. We also noted the ability to separate enantiomers using chiral chromatography. In this installment, we’ll talk about the first steps in developing an HPLC method that will separate chiral compounds of interest.
With any approach to chiral chromatographic method development, we begin by selecting a column. The column is the single most important factor in the ultimate success of our separation, so we want to choose wisely! Unfortunately, it can be difficult to predict which chiral stationary phase (CSP) will resolve our specific compounds. As a first step, it’s a good idea to check the applications database since it’s possible that we’ve already identified a set of conditions (CSP, mobile phase composition, flow rate, etc.) that works well. But even if we don’t have a method for the exact compound, we might find a close analogue that will give us a good idea of where to start.
If we’re starting from scratch, we’ll want to try the separation using several columns. Regis Technologies offers a number of versatile CSPs including polysaccharide-based, Pirkle-type, and crown ether phases. Let’s take a moment to look at these different types of CSPs.
Polysaccharide-based Phases
Polysaccharide-based CSPs feature derivatized cellulose or amylose polymers. Their usefulness as chiral selectors is due in large part to the stereochemistry of the individual glucopyranose units and the conformational chirality imparted by the helical twists of their polymeric backbones. Derivatization influences the polymer conformations and provides addition points of interaction with analytes to influence selectivity.
Historically, coated phases are very popular and among the most selective CSPs available. But since they are coated and not covalently bonded to the surface of the silica particles, care must be taken to avoid mobile phases that can solubilize the polymers and potentially damage the column.
Immobilized polysaccharides are prepared similarly to the coated phases but are crosslinked to provide additional stability and solvent compatibility. Mobile phases that contain dichloromethane, chloroform, ethyl acetate, THF, MTBE, etc. are perfectly safe to use with immobilized polysaccharide phases.
Both coated and immobilized versions are quite versatile. They can be used with great success in normal phase, reversed-phase, and supercritical fluid modes of chromatography.
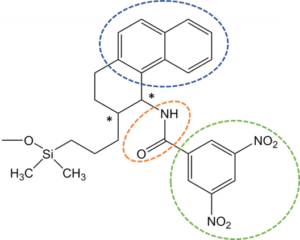
Figure 1 – Whelk-O® 1; π-electron donating, π-electron-accepting, and H-bonding moieties are circled in blue, green, and orange respectively.
Pirkle-Type Phases
Pirkle-type (brush-type) phases are low-molecular weight, synthetic selectors that are designed to target certain types of interactions with chiral analytes. Because they are covalently bonded to the silica particles, there are no solvent compatibility concerns. In many cases they are available in two absolute stereoconfigurations, allowing you to swap the elution order of enantiomers simply by changing the column.
Whelk-O® 1, available in (R,R)- and (S,S)-, is one of the most popular Pirkle-type selectors. As shown in Figure 1, this versatile CSP incorporates π-electron donor and π-electron acceptor regions and an amide hydrogen donor-acceptor. These moieties provide the points of simultaneous interaction that are necessary to separate enantiomers.
Crown-ether Phases
Crown ether phases like ChiroSil® and ChiroSil® ME consist of macrocyclic polyethers with a central cavity of certain size. The electron-donating oxygens within the cavity contribute toward hydrogen bond formation with ionized ammonium groups. These phases are especially discriminating for primary and secondary amines, including many underivatized amino acids. They are most effective when using acidic aqueous mobile phases.
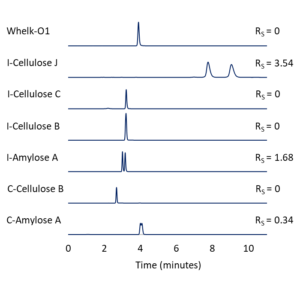
Figure 2 – Differences in resolution (RS) of EPN enantiomers on several different Regis CSPs. All columns are 25 cm x 4.6 mm, 5-µm. MP = (80/20 vol/vol%) hexane/ethanol; Flow = 1.5 mL/min.
CSP Screening
To determine which CSP is best capable of resolving our chiral compounds of interest, it is good practice to screen several of them with a few different mobile phase conditions. As an example, let’s consider the screening process as it might look for a normal phase separation of the organothiophosphate insecticide, EPN. If we start with Reflect™ C-Amylose A, we can begin our screening work by finding a mobile phase that will elute the compound with a retention factor (k’) between 1 and 5.
We can start with a relatively strong mobile phase like (50/50 vol/vol%) hexane/ethanol. If our compounds elute too early, we can increase the proportion of the weak component, hexane. (80/20 vol/vol%) hexane/ethanol provides an appropriate amount of retention, but the resolution of the enantiomer peaks is only RS = 0.34. As shown in Figure 2, if we use the same mobile phase conditions to screen several additional CSPs, Reflect™ I-Cellulose J provides good retention and resolution (RS = 3.54).
In some cases, none of the columns we try will provide adequate separation with our initial mobile phase system. A simple swap of isopropanol for ethanol might make all the difference, as illustrated in Figure 3. In (80/20 vol/vol%) hexane/ethanol, Reflect™ I-Cellulose C provides no observable enantiomeric resolution of the herbicide diclofop-methyl. With (80/20 vol/vol%) hexane/isopropanol, baseline separation is achieved. Seemingly small changes in mobile phase composition can result in big selectivity differences. We often screen both hexane/ethanol and hexane/isopropanol when looking for a good normal phase HPLC method.
From preliminary data like this, it is often possible to determine which chiral stationary phases should be taken on for further method development in order to optimize the resolution, peak shapes, analysis time, etc..
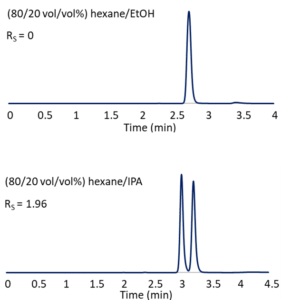
Figure 3 – The influence of mobile phase on selectivity. Column: Reflect™ I-Cellulose C, 25 cm x 4.6 mm, 5-µm; Flow: 1.5 mL/min.
Coming Soon
In our next blog, we’ll look at some additional variables aside from the CSP that can have important influence on chiral separations.
Did you miss part one in the Chiral Method Development series? You can read it here.
Additional Resources
- Download or request a copy of our Chiral Handbook for HPLC and SFC applications here.
- Download or request a copy of our HPLC and SFC Method Development poster here.
- Learn more about Regis Technologies Whelk-O®, Reflect™, and other Chiral HPLC & SFC Columns.
- Check out our chiral database with over 900 chiral applications.
- Learn about Regis’ Try & Buy column program and how you can try a Regis HPLC or SFC column risk free.
- Visit our Literature Library to download product information, application notes, and other technical resources.