Mastering ICH Q14: What You Need To Know


In this article, we will be taking a deep dive into ICH Q14. Some of the topics that we’ll cover include the role of ICH Q14 and the main elements of analytical procedures. We’ll also detail exceptions and challenges. By the end of this article, you should have a better understanding of ICH Q14 and feel more informed on this topic moving forward.
Overview of ICH Q14
ICH Q14 helps detail how to maintain analytical procedures for assessing the quality of drug products and substances, and provides a harmonized guideline on analytical method development, all while facilitating a risk-based approach. This provides scientifically sound analytical method design with respect to the individual process.
Additionally, ICH Q14 governs a control strategy approach to method development to maintain procedures suitable for the analysis of drug substances and drug products. ICH Q14 also ensures the quality and safety of pre-approved and post-approved drug products and substances so they are suitable and ready to go to market.
Main Elements of ICH Q14
There are multiple key elements of ICH Q14 that must be addressed in order to fully understand its importance in the drug development process. The first element would be Principles of Analytical Method Development. When talking about Principles of Analytical Method Development, material specifications need to be considered, such as regulatory requirements, dosage form, and drug substance versus drug product.
Another significant element of ICH Q14 is Analytical Method Development Process. With this element, it’s paramount to understand the Analytical Target Profile (ATP). This includes understanding Critical Quality Attributes (CQAs), as well as Method Performance Characteristics (the process of optimizing for method in preparation of Analytical Method Validation). Additionally, it’s important to note that with Analytical Method Validation, testing will need to be performed for the following parameters:
- Linearity
- Accuracy
- Specificity
- Range
- Precision
- LOQ
- LOD
- Robustness
- Forced Degradation
Another key element of ICH Q14 is Lifecycle Management. With Lifecycle Management, an evaluation will be performed to check for any changes in process post approvals. Then, if changes are detected, revalidation may occur. Please see the flowchart below for an in-depth look into the Analytical Procedure Lifecycle.
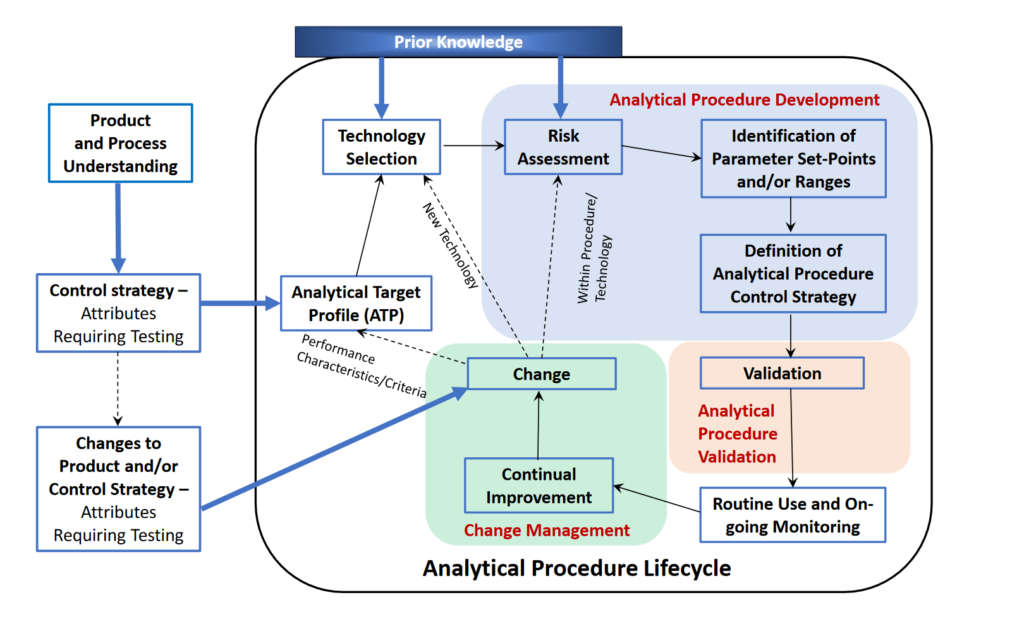
(2022). ANALYTICAL PROCEDURE DEVELOPMENT ICH Q14 [Photograph], page 4. https://database.ich.org/sites/default/files/ICH_Q14_Document_Step2_Guideline_2022_0324.pdf
Exceptions and Challenges
When it comes to mastering ICH Q14, you’ll need to keep in mind that you may end up facing some exceptions and challenges along the way. It’s important to monitor what’s happening during each step of the process so you remain aware of any challenges and can make adjustments as needed. Recognizing these challenges can help you prepare and understand what needs to happen going forward to ensure success.
When it comes to exceptions and challenges during the development process, the complexity of methods may come into play. In this case, there will be ATP changes to monitor. Another thing to be aware of is regulatory compliance. Analytical Method Design could be impacted if there are various changes to other harmonized chapters. Additionally, if you find that the procedure is resource intensive, you will need to conduct monitoring of processes, methods, and compliance requirements continuously.
Conclusion

There are a great number of things to learn when it comes to ICH Q14, including that it harmonizes several chapters. Some of these chapters include ICH Q2, ICH Q8, and ICH Q12 (amongst other chapters). ICH Q14 has also given instructions for control strategy, key development considerations, and making risk-based approaches for pre-approved and post-approved drug products and substances. Overall, ICH Q14 can be an incredible resource to reference during the drug development process. This method can also help you make informed decisions throughout the drug development cycle.
If you would like to learn more about how our team of professional process chemists can help your company bring a new drug to market, please contact us. A member from our knowledgeable team would be pleased to learn more about your company and share ideas on how we can provide assistance.