Process Development: A Systematic Approach


Introduction
Welcome to the second part of our three-part blog series. If you haven’t read the first part of this series, The Role of Process Chemistry, you may do so by visiting our blog. In this article, we will share a systematic approach to process development and what this means for a process chemist. We will cover everything from the initial approach, to what happens during late-stage development, and beyond.
The Initial Approach
The Initial Approach is an essential part of process development. During this stage, a vial scale is utilized (note that the vial scale is not useful for robustness, yield, or consistency), and many experiments are conducted to screen reactions and work-up conditions. Additionally, solubility information is gathered in great detail, and a reaction profile is created to share information about impurities.
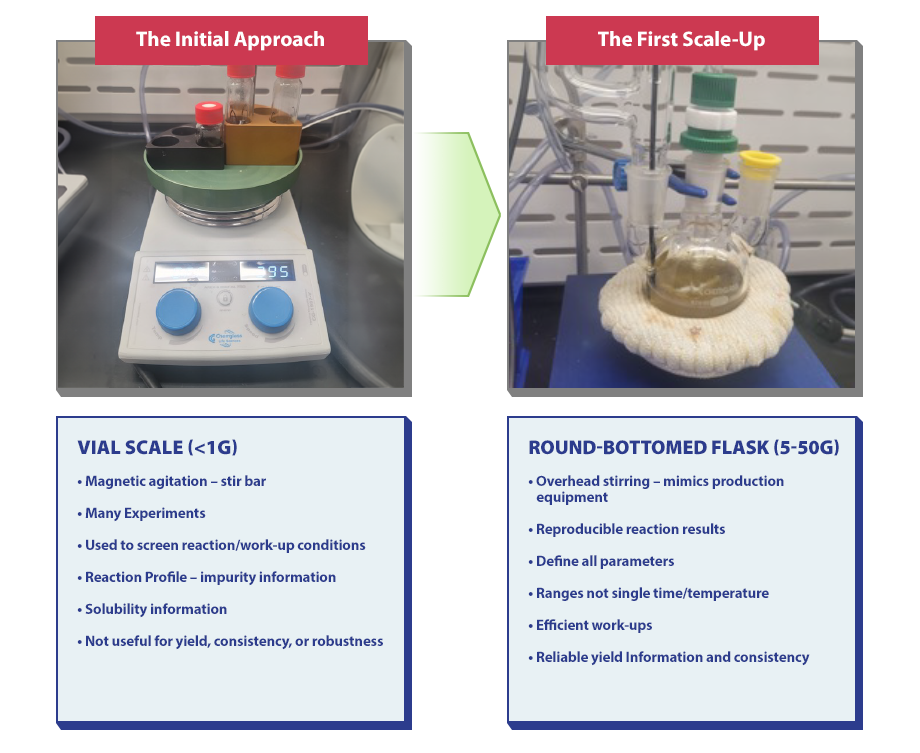
The First Scale-Up
During this stage, a round-bottomed flask (5-50g) is used to provide reproducible reaction results, define all parameters, offer efficient work-ups, and provide reliable yield consistency and information. The EasyMax Machine is also used during this stage and possesses features such as calorimetry, programmable temperature plans, slow reagent/solvent additions, tight temperature control, and crystallizations.
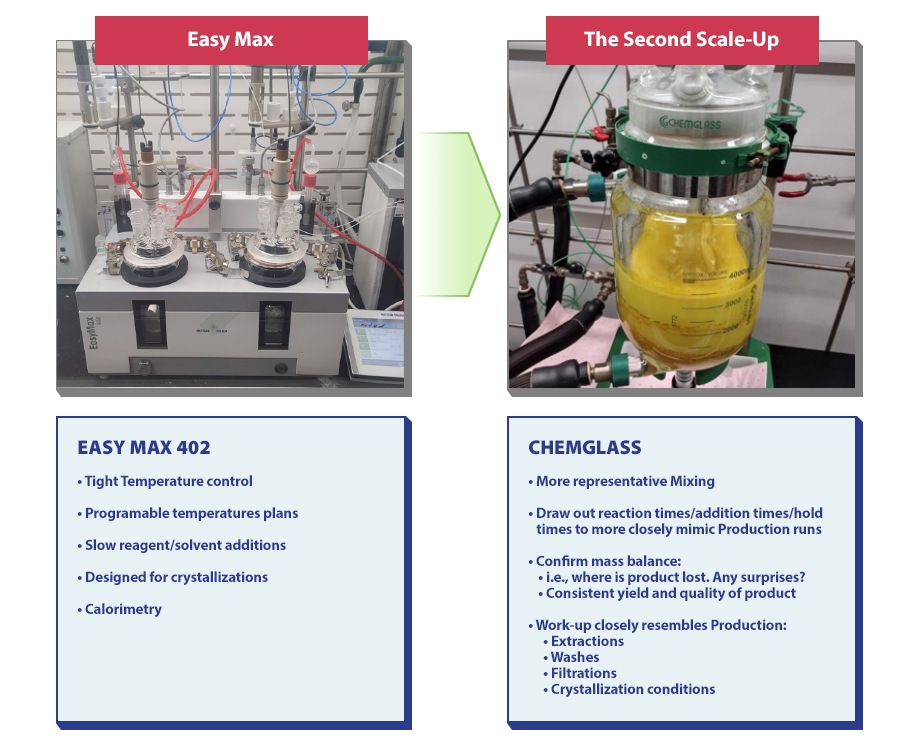
The Second Scale-Up
There are several, significant steps taken during the Second Scale-Up that help the process chemist better understand what is happening at this stage. The mass balance will be confirmed, the consistent yield quality of the product will be checked, representative mixing will occur, and reaction, addition, and hold times will be drawn out to mimic production runs. Another factor worth noting is that the work-up resembles production and includes washes, filtrations, extractions, and crystallization conditions.
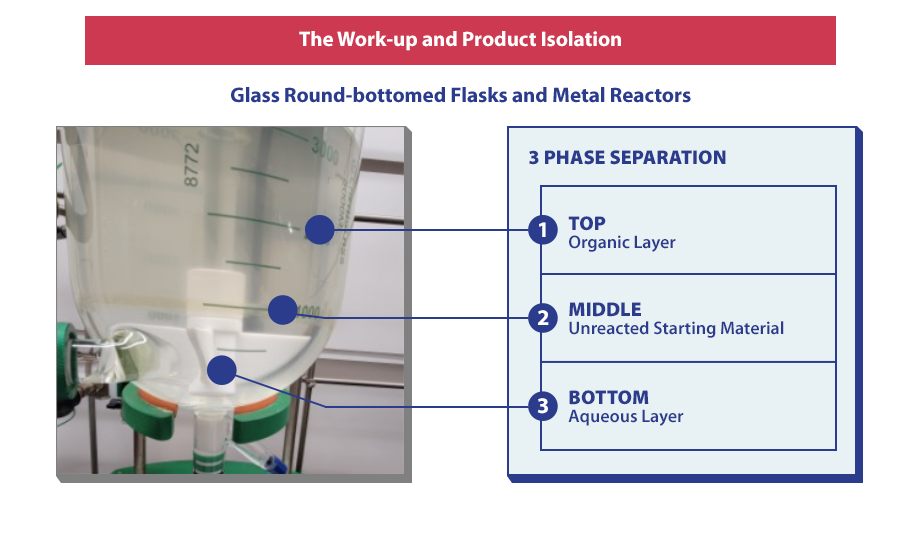
The Work-Up and Product Isolation
Glass round-bottomed flasks and metal reactors are used during this point of the process and also include 3-phase separation. The goal of the Work-Up and Product Isolation stages is to efficiently get the product out of the reaction solution. This can be done by removing impurities, ensuring the product is in the correct solid state form, developing isolation and drying conditions, and confirming that there are solids and not oils.
However, there are some problems commonly encountered during the Work-Up and Product Isolation stages, including multiple extractions, emulsions, slow filtration, and moving a viscous oil from one vessel to another. These problems are seen frequently by process chemists and may cause issues later on if they are not monitored and corrected.
What a Systematic Approach Means to a Process Chemist
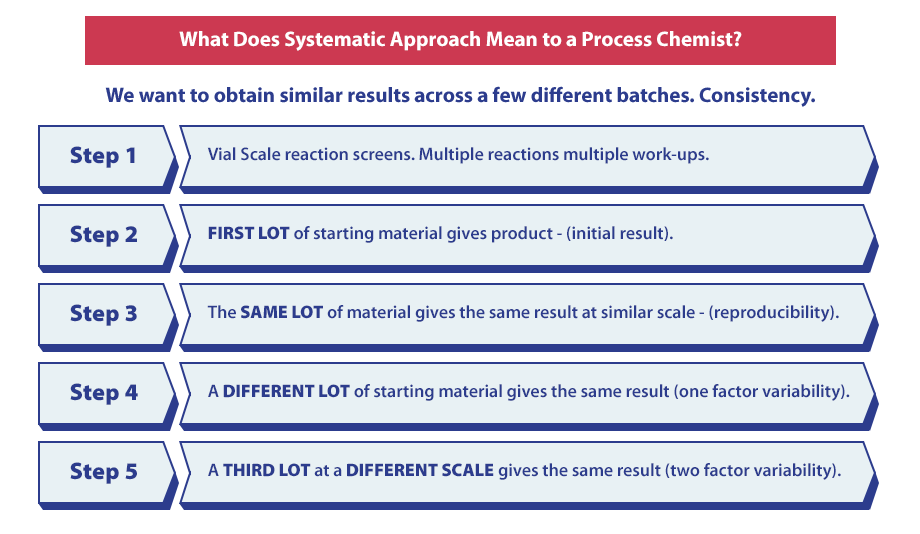
The goal of a systematic approach is to find consistency in results across multiple batches, which can be done utilizing the following five steps:
- Step 1: Vial scale reaction screens and multiple reactions with multiple work-ups.
- Step 2: The first lot of starting material gives product.
- Step 3: The same lot of material gives the same result at a similar scale.
- Step 4: A different lot of starting material gives the same result.
- Step 5: A third lot at a different scale gives the same result.
The Purpose of Hold Points and Stress Tests
Hold Points and Stress Tests play a significant role in process development,as they force the process outside the normal operating ranges (robustness) and ensure that the product is stable at various points. Furthermore, Hold Points and Stress Tests give the process chemist some peace of mind by providing answers to any questions they may have.
The Lab Confirmation Batch
The Lab Confirmation Batch stage is the initial practice run and the first time a process goes from beginning to end with only slight adjustments. All of the changes that the process chemist made will be tested during this time. This stage is a joint effort and includes solid state chemistry, tech-ops, analytical development, and process safety.
The Purpose of a Demo Batch
A demo batch plays an essential role in process development and always takes place after Lab Confirmation. A demo batch tests the scalability of the process and is non-GMP. It’s important to note that the scale of this batch is usually 1/10 of the scale of planned production batches. Further, the lab confirmation and demo batch results are used to set specifications for future production batches.
The Role of a Process Chemist During a Production Batch
Process chemists play an essential role during a production batch and ensure that everything is running smoothly and without interruption. They are present throughout the duration of the batch and are closely involved in each stage. If any issues arise during a Production Batch, the process chemist will work with Production staff, Process Safety and Tech-Ops to assess the situation, gather information and devise a data-driven solution.
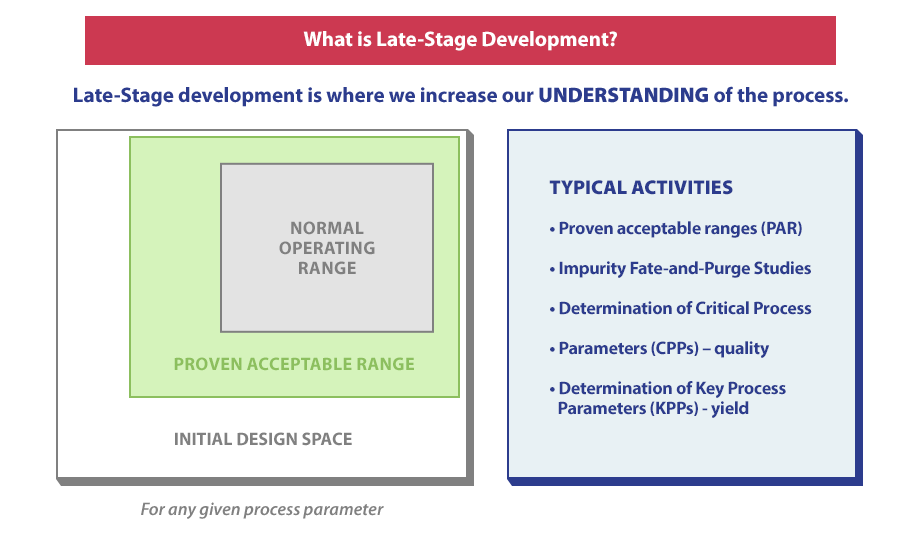
What Happens During Late Stage Development
Late Stage Development helps the chemist better understand the process. During this stage, the process chemist will check if process parameters are in the normal operating range or the proven acceptable range. Some activities that take place during Late Stage Development include:
- Proven acceptable ranges (PAR)
- Impurity FATE-and-Purge Studies
- Determination of Critical Process Parameters (CPPs)
- Determination of Key Process Parameters (KPPs)
Conclusion
Thank you for taking the time to read part two of our three-part series. In part three, we will go over case study summaries. Please continue to check our blog for the latest updates, or follow our company page on LinkedIn for more insider news.
If you want to learn more about how our team of experienced process chemists can help your company bring a new drug to market, please contact us. A knowledgeable member of our team would be happy to learn more about your company and share how we could be of value to you.
If you missed part one of the The Role of Process Chemistry Series, you can read it here.