Getting Started with Chiral Method Development Part Three: Method Development Optimization


In Part 2 of our series on Chiral Method Development, we discussed the features of several different types of chiral stationary phases (CSPs). We also discussed a general approach to column and mobile phase screening, which can be helpful for identifying candidates to carry on for further method development and optimization. While column stationary phase and mobile phase composition are the most important factors in developing a successful chiral separation, additional conditions can be investigated in our efforts to improve a separation in terms of speed, peak shape, or resolution. In this installment, we look at several of those conditions.
Mobile Phase Additives
As we discussed previously, simple adjustments to mobile phase strength or composition can often make big differences in retention and chiral resolution. There are some situations,

Figure 1 – The separation of ketorolac on Reflect C-Amylose A with (a) no acidic additive, (b) 0.1% TFA, and (c) 0.1% acetic acid. The inclusion of an acidic additive in the mobile phase can improve peak shape for acidic samples.
though, where retention may be perfectly acceptable (e.g. 1≤ k’ ≤ 5) and resolution is clearly apparent, but peak shape is unacceptable. A simple mobile phase additive might really improve the situation.
Figure 1 shows an example of this using racemic ketorolac, a carboxylic acid-containing molecule with a pKa of ~ 3.5. In a normal phase separation performed on Reflect C-Amylose A in (50/50 vol/vol%) hexane/ethanol, the enantiomers are resolved, but peak shape is quite poor. The addition of 0.1 vol% of either TFA or acetic acid to the mobile phase improves both peak shape and resolution. In cases like this, the exact amount of acid is not especially critical, and 0.1% is usually enough to suppress ionization of the carboxylic acid and to limit unfavorable interactions between the analyte and stationary phase.
Separations involving basic samples can be approached similarly, though instead of adding an acidic component, a basic additive like 0.1% diethylamine is used to overwhelm the undesired interactions between the basic analyte and silica particles that lead to poor peak shape.
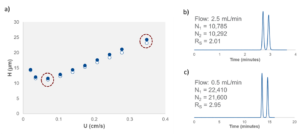
Figure 2 – (a) Plate height (H) vs mobile phase linear velocity (U) for the separation of 1-acenaphthenol on Reflect I-Cellulose C. Chromatograms highlight the enantioresolution values (RS) when the separation is performed at (b) 2.5 mL/min and (c) 0.5 mL/min. L = 25 cm, i.d. = 4.6 mm, dp = 5-µm, MP = (90/10 vol/vol%) hexane/isopropanol.
Flow Rate
For certain difficult separations where there is little space between our peaks of interest, it can be worthwhile to investigate the effect of flow rate on the quality of the separation. Column performance, in terms of plate height vs. mobile phase linear velocity, is very often qualitatively similar to the plot shown in Figure 2. Looking at this plot, we see that plate height (H) varies depending on the flow rate at which we run the column. At very low and very high flow rates, plate heights are relatively high, meaning that the total column efficiency (i.e. the theoretical plate count) is lower, and peak resolution values suffer. At some intermediate value, however, we find an optimum flow rate where our column provides the maximum number of theoretical plates and our peaks are best resolved. This optimum flow rate is dependent on several factors including column dimensions, particle size, mobile phase viscosity, analyte diffusion properties, and the nature of the interactions between the sample and the CSP.
In Figure 2, we can see the effects of running racemic 1-acenaphthenol on Reflect I-Cellulose C at several different flow rates. At 2.5 mL/min, column efficiency (N) is approximately 10,500 plates and the enantioresolution value is 2.0. At the more optimal flow rate of 0.5 mL/min, N = 22,000 and resolution increases to approximately 3.0.
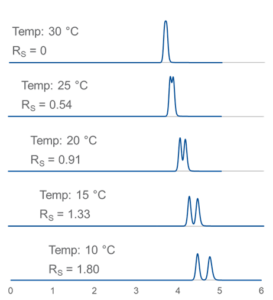
Figure 3 – Decreasing column temperature improves the resolution of racemic 1-acenaphthenol on Reflect C-Amylose A. L = 25 cm, i.d. = 4.6 mm, , dp = 5-µm, MP = (80/20 vol/vol%) hexane/ethanol; F = 1.5 mL/min.
Temperature
Precise control of column temperature is an important factor in HPLC method robustness. Temperature-control can also be used to influence the enantioselectivity of a chiral separation by affecting the nature of the analyte-mobile phase-stationary phase
interactions. As shown in Figure 3, lower temperatures generally lead to better chiral selectivity. But it can sometimes be difficult to predict exactly how temperature will influence a given separation. It is worthwhile to explore a range of oven temperatures in the course of method development. Depending on instrument capability and the solvent system employed, temperatures as low as 0°C can be used. We recommend that you not exceed 40°C when using Reflect polysaccharide-based phases or 60° C when using Pirkle-type phases, like Whelk-O1.
Temperature can also be used to diagnose whether on-column racemization is complicating a particular chiral separation. The interconversion of two isomeric species is often characterized by two distinct peaks with an elevated, plateau-like region between them, as shown in Figure 4. If this phenomenon is observed, try running the separation at lower temperatures where such interconversion may be suppressed. If the racemization cannot be sufficiently mitigated by temperature alone, it will be useful to investigate non-alcoholic strong modifiers.
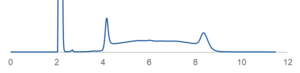
Figure 4 – Chromatogram showing an example of on-column racemization. Peaks at approximately 4 and 8 minutes represent the characteristic elution times of the two enantiomers. The elevated signal between the peaks is a typical indicator of isomerization.
Conclusion
When developing a new chiral separation, there are so many column chemistries, mobile phase compositions, and additional instrument parameters to consider that the task can seem overwhelming. Fortunately, at Regis, we have a number of resources to help you get started. Our chiral database has hundreds of applications to assist you in finding separation conditions for samples that might be similar to your compounds of interest. Additionally, our free chiral screening service can help you to identify promising column chemistries and mobile phases that are worthy of further evaluation. Then, once you have these essential factors in place, you can feel free to explore additional parameters like the ones described above to optimize your method in proportion to your chromatographic needs. And if you ever run into difficulties, feel free to reach out to the chiral experts at Regis Technologies. We’re here to help!
In case you missed our previous blogs in the Method Development Series, be sure to read part one and part two.
Additional Resources from Regis Technologies
- Download or request a copy of our Chiral Handbook for HPLC and SFC applications here.
- Download or request a copy of our HPLC and SFC Method Development poster here.
- Learn more about Regis Technologies Whelk-O®, Reflect™, and other Chiral HPLC & SFC Columns.
- Check out our chiral database with over 900 chiral applications.
- Learn about Regis’ Try & Buy column program and how you can try a Regis HPLC or SFC column risk free.
- Visit our Literature Library to download product information, application notes, and other technical resources.